Strategic Deviation & CAPA Management: From Compliance to Quality Maturity
A deviation is more than a non-conformity—it is a signal from your process. We provide the technical expertise to decode these signals through forensic Root Cause Analysis, ensuring that your CAPA is not just a regulatory requirement, but a permanent resolution. 30 years of inspection-proven precision.
System Readiness
Establishing an objective baseline. We build risk-based triage models that eliminate subjectivity according to ICH Q9(R1).
Backlog & Recovery
Rehabilitating ineffective systems. We perform forensic reviews of open deviations to clear backlogs and identify hidden systemic risks.
Quality 4.0 Evolution
Designing scalable systems—from lean paper-based SOPs to digital eCAPA platforms validated under CSA and GAMP.
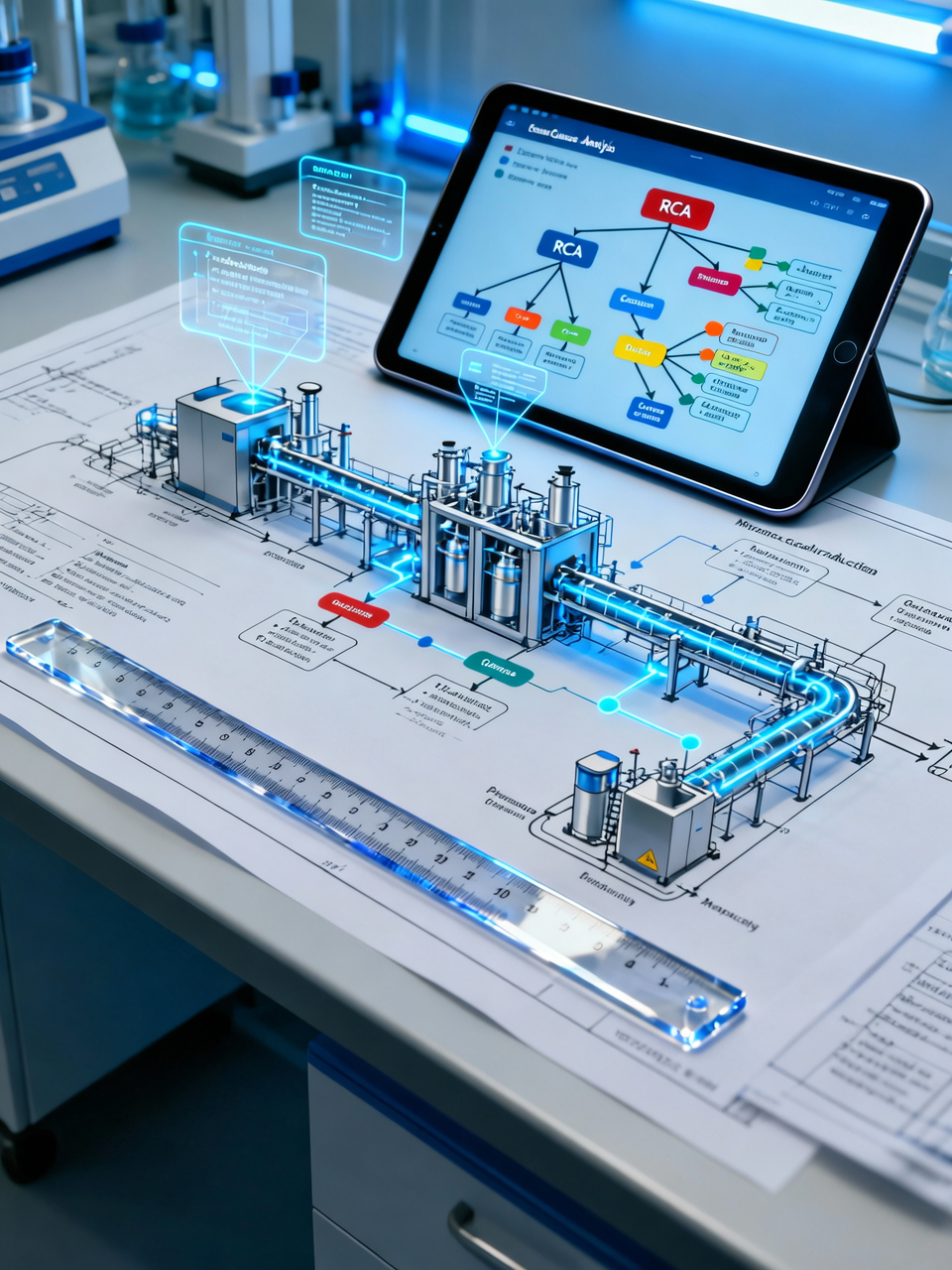
Robust Deviation Management: Decoding the Signal
A deviation is an unplanned departure from a validated state. At Schmidt-GMP, we treat the "Human Error" trap as the beginning of an investigation, not the endpoint.
The "Golden Start": Critical actions taken in the first 24 hours to preserve evidence and gather unbiased witness testimonies through Gemba walks.
Forensic Root Cause Analysis (RCA): Utilizing the SRK Model (Skills, Rules, Knowledge) and GEMS to distinguish between individual slips and systemic failures.
Managing Subjectivity: Explicit documentation of assumptions to mitigate Anchoring Bias and ensure defensible regulatory outcomes.
“A deviation is an observation; a CAPA is a commitment. In 30 years of inspections, I have learned that the quality of your CAPA system is the truest reflection of your company’s Quality Management Maturity (QMM).”
CAPA Management: The Blueprint for Permanent Resolution
While Deviation Management identifies the event, the CAPA Subsystem provides the mechanism for systemic resolution.
Systemic Escalation: Not every deviation requires a CAPA. We implement risk-based filters to ensure major, critical, or recurring events are escalated for deep-dive remediation.
Effectiveness Verification (VOE): Moving beyond "SOP updates and Retraining". We design SMART effectiveness checks (Specific, Measurable, Achievable, Relevant, Time-bound) to confirm the problem is truly solved.
Predictive Trends: Integrating AI-Agentic tools to surface patterns in legacy data, predicting potential failures before they manifest as deviations.